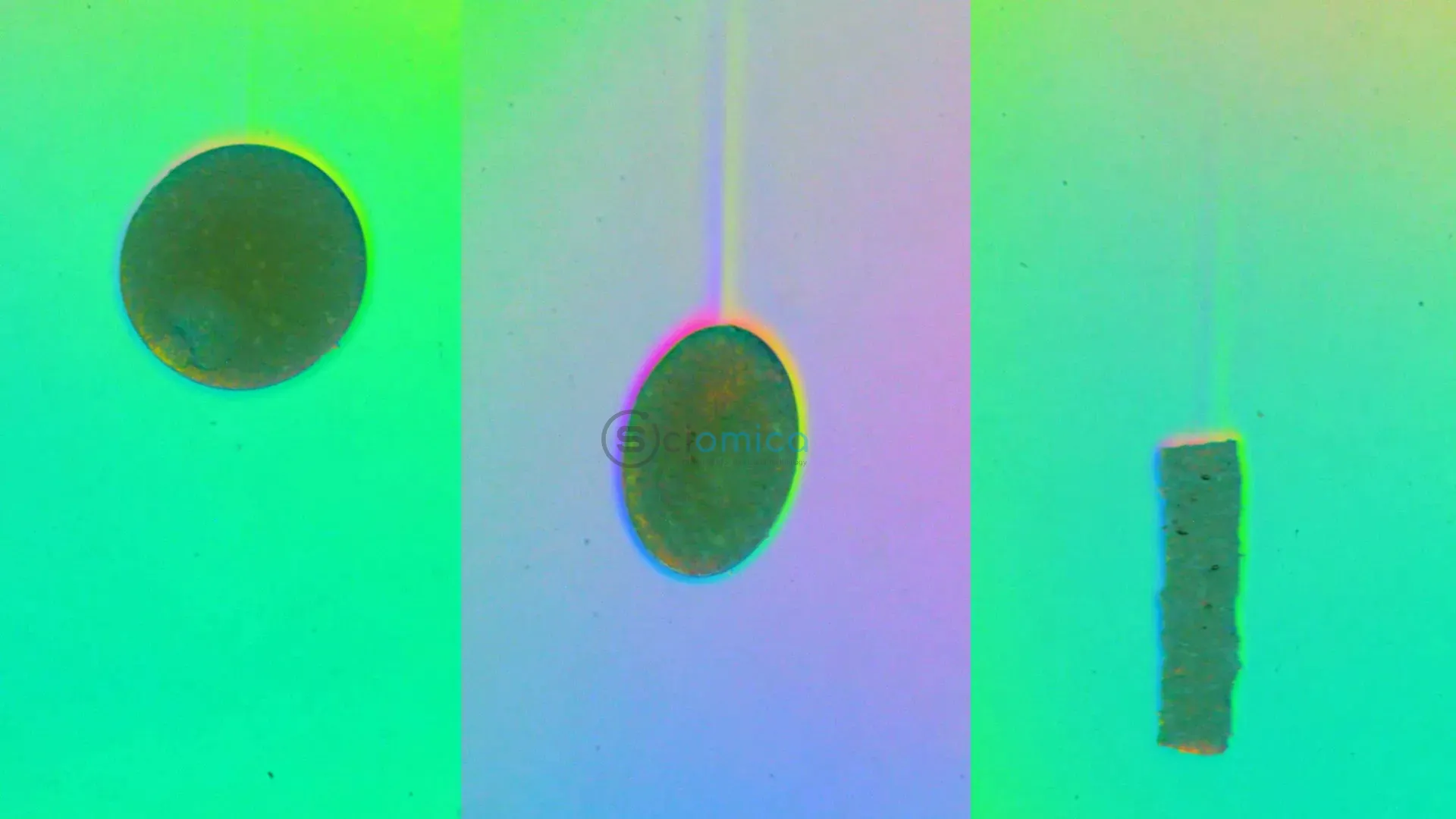The deep ocean often resembles a stunning snow globe, where organic particles from plant and animal matter drift from the surface, settling below and forming what is known as “marine snow.” This mesmerizing phenomenon is essential for cycling carbon and nutrients throughout the world’s oceans. Recent research conducted by experts from Brown University and the University of North Carolina at Chapel Hill has uncovered new insights into the dynamics of particle sinking in stratified fluids, such as oceans, where fluid density varies with depth.
In a groundbreaking study published in the Proceedings of the National Academy of Sciences, researchers revealed that the sinking speed of particles is influenced not just by drag forces from the surrounding fluid, but also by the ability of the particles to absorb salt in relation to their volume. Robert Hunt, a postdoctoral researcher at Brown, who led the study, remarked that surprisingly, smaller particles can sink faster than larger counterparts. This finding contradicts conventional expectations in fluids with uniform density.
The implications of this research could significantly enhance our understanding of ocean nutrient cycling and the behavior of porous particulates, including microplastics. Daniel Harris, an associate professor at Brown and overseer of the project, stated that they developed a straightforward formula allowing for easy predictions of sinking speeds by inputting different parameters, such as particle size and the rate of liquid density change. “There’s value in having predictive power that’s readily accessible,” he emphasized.
The study originated from earlier investigations by Hunt and Harris into neutrally buoyant particles — those that sink to a specific depth and then remain suspended. During these observations, Hunt encountered unexpected behavior, where particles that were presumed to be neutrally buoyant were continuously sinking, prompting further inquiry into their characteristics.
This led to the formulation of a new theoretical model, demonstrating that the capacity to absorb salt directly affects sinking rates. The model suggests that the more salt a particle absorbs relative to its size, the quicker it sinks, a conclusion that intriguingly posits that smaller porous particles descend faster than larger ones.
To validate their model, the team designed a linearly stratified water setup, allowing them to control the liquid density at varying depths. By mixing water from fresh and saltwater sources, they engineered a large tank with a gradient density profile. They utilized 3D-printed molds to create particles of various sizes and shapes from agar, a gelatinous substance derived from seaweed, and documented their sinking behavior with the help of cameras.
The experimental outcomes supported their predictions, revealing that smaller spherical particles indeed sank quicker, and that for elongated or flatter particles, their smallest dimension dictated their sinking speed. This indicates that elongated particles may descend faster than spherical ones of equal volume.
These findings, the researchers stated, hold substantial promise for gaining insights into the settling behavior of particles within more intricate ecological contexts, whether that be for natural carbon cycling or engineering methods to expedite carbon capture in sizable aquatic environments. Harris noted the team’s aim to simplify complex phenomena to grasp fundamental physics before collaborating with field researchers to apply these insights in real-world scenarios.
In collaboration with co-authors Roberto Camassa and Richard McLaughlin from UNC Chapel Hill, this work was bolstered by funding from the National Science Foundation and the Office of Naval Research, emphasizing the broader implications of this research for oceanographers and climate scientists.
Reference:
- Robert Hunt, Roberto Camassa, Richard M. McLaughlin, Daniel M. Harris. Diffusion-limited settling of highly porous particles in density-stratified fluids. Proceedings of the National Academy of Sciences, 2025; 122 (25) DOI: 10.1073/pnas.2505085122