In a groundbreaking development for type 1 diabetes treatment, a clinical trial involving a dozen volunteers demonstrated significant improvements in their condition after undergoing a novel stem cell therapy. Over the course of a year, all but two participants were able to eliminate their reliance on insulin therapy, marking a potential turning point in diabetes management.
The phase 1/phase 2 clinical trial, conducted by researchers at the University of Toronto, has garnered attention for its promising results. This glimpse of hope is paramount for the approximately 8.4 million individuals globally living with type 1 diabetes, a severe autoimmune disorder where the immune system attacks insulin-producing cells in the pancreas.
The innovative therapy, developed by Boston-based Vertex Pharmaceuticals, raises the possibility of restoring the body’s capacity to produce insulin through stem cell-derived islet cells. While further studies are essential to evaluate the long-term effectiveness of this treatment, the results thus far are encouraging.
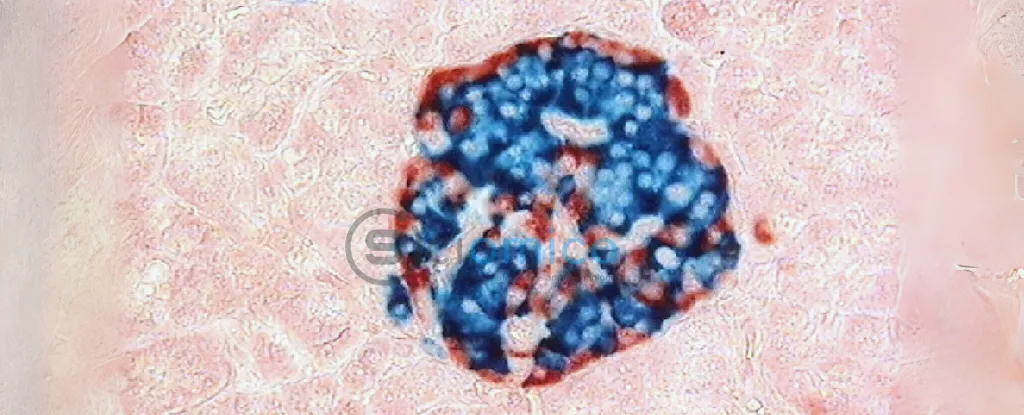
Participants in the trial, all diagnosed with severe type 1 diabetes, received infused islet cells derived from human stem cells in a treatment referred to as zimislecel. Along with the infusion, patients underwent immunosuppressive therapy before and after their treatment to enhance the likelihood that the new islet cells would function effectively and not be rejected by the body.
For individuals with type 1 diabetes, managing insulin levels is an ongoing struggle. They face a tenuous balance: insufficient insulin can lead to dangerously high blood sugar levels and resultant organ damage, while excessive insulin can plunge them into hypoglycemia, potentially causing seizures, loss of consciousness, or even death. Typically, patients rely on insulin injections to maintain this balance, complicating daily life.
Historically, pancreas transplants and donor islet cell transplants have shown promise, but they require multiple donors and come with several challenges, including organ availability and transplant rejection risks. The study’s lead surgeon, Trevor Reichman, expressed optimism about the results, stating, “These findings showed that zimislecel islet cells were functional and self-regulated appropriately.” The induced islet cells not only produced insulin but did so at stable levels sufficient to decrease the patients’ need for external insulin doses.
While the trial did report some mild to moderate side effects related to the immunosuppressive therapy, including decreased kidney function, researchers noted that no serious adverse events were connected specifically to the new islet cell treatment. Unfortunately, two participants did lose their lives during the trial: one due to post-surgical infection and another linked to an unrelated health condition.
The encouraging results from this trial have paved the way for advancements into phase 3 clinical trials, with researchers now gathering evidence that supports the feasibility of producing pancreatic islets from pluripotent stem cells for the treatment of type 1 diabetes.
This promising research has been published in the New England Journal of Medicine, contributing valuable insights to the ongoing quest for effective diabetes therapies.